作者:
Sidra Zafar(医学博士)是费城威尔斯眼科医院视网膜科的玻璃体视网膜外科研究员。她在约翰・霍普金斯大学医学院威尔默眼科研究所完成了住院医师培训,在为期两年的研究员培训结束后,她将返回该机构担任科室副主任。
Sunir Garg(医学博士,美国外科医师学会会员,美国视网膜专家学会会员)是威尔斯眼科医院视网膜科的主治医师。他的临床研究方向包括糖尿病视网膜病变、年龄相关性黄斑变性、复杂性视网膜脱离、视网膜血管疾病及眼部炎症的先进治疗方法。Garg 医生著作颇丰,还是3本医学教科书的主编。
参考文献
1. Fleckenstein M, Schmitz-Valckenberg S, Chakravarthy U. Age-related macular degeneration: A review. JAMA. 2024;331(2). doi:10.1001/jama.2023.26074
2. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmol. 2013;120(4). doi:10.1016/j.ophtha.2012.10.036
3. Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5). doi:10.1016/S0039-6257(05)80092-X
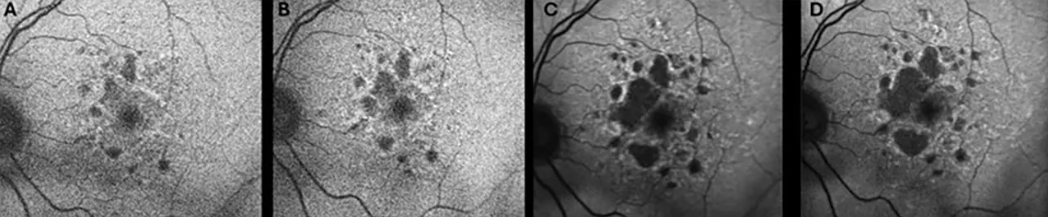
4. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmol. 2018;125(3):369-390. doi:10.1016/j.ophtha.2017.08.038
5. Chakravarthy U, Bailey CC, Johnston RL, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmol. 2018;125(6):842-849. doi:10.1016/j.ophtha.2017.11.036
6. Holz FG, Strauss EC, Schmitz-Valckenberg S, Van Lookeren Campagne M. Geographic atrophy: Clinical features and potential therapeutic approaches. Ophthalmol. 2014;121(5). doi:10.1016/j.ophtha.2013.11.023
7. Boyer D, Hu A, Warrow D, et al. LIGHTSITE III: 13-month efficacy and safety evaluation of multiwavelength photobiomodulation in nonexudative (dry) age-related macular degeneration using the Lumithera Valeda light delivery system. Retina. 2024;44(3). doi:10.1097/IAE.0000000000003980
8. Pavluk L, Kaya M. FDA approves SYFOVRETM (pegcetacoplan injection) as the first and only treatment for geographic atrophy (GA), a leading cause of blindness. February 17, 2023. www. https://investors.apellis.com/news-releases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only. Accessed June 23, 2025.
9. Iveric Bio receives U.S. FDA approval for IZERVAYTM (avacincaptad pegol intravitreal solution), a new treatment for geographic atrophy. https://www.astellas.com/en/news/28281 Accessed June 23, 2025.
10. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. The Lancet. 2023;402(10411). doi:10.1016/S0140-6736(23)01520-9
11. SYFOVRE® (pegcetacoplan injection)continued to demonstrate increasing treatment effects over 3 years in patients with geographic atrophy (GA). November 4, 2023. https://investors.apellis.com/news-releases/news-release-details/syfovrer-pegcetacoplan-injection-continued-demonstrate-0. Accessed June 23, 2025.
12. SYFOVRE® (pegcetacoplan injection) preserved visual function at 36 months in GALE extension study in geographic atrophy (GA). June 10, 2024. https://investors.apellis.com/news-releases/news-release-details/syfovrer-pegcetacoplan-injection-preserved-visual-function-36. Accessed June 23, 2025.
13. Witkin AJ, Jaffe GJ, Srivastava SK, Davis JL, Kim JE. Retinal Vasculitis After Intravitreal Pegcetacoplan: Report from the ASRS research and safety in therapeutics (ReST) committee. J Vitreoretin Dis. 2024;8(1). doi:10.1177/24741264231220224
14. Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal 2/3 trial. Ophthalmol. 2021:128 (4)576-586. Vol 128. ; 2021. doi:10.1016/j.ophtha.2020.08.027
15. Patel SS, Lally DR, Hsu J, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye (Basingstoke). 2023;37(17). doi:10.1038/s41433-023-02497-w.
16. Khanani AM, Patel SS, Staurenghi G, et al. Efficacy and safety of avacincaptad pegol in patients with geographic atrophy (GATHER2): 12-month results from a randomised, double-masked, phase 3 trial. The Lancet. 2023;402(10411). doi:10.1016/S0140-6736(23)01583-0
17. Jaffe GJ, Khanani AM. Two studies to learn if avacincaptad pegol works and is safe in people with geographic atrophy: a plain language summary of the GATHER1 and GATHER2 studies. Immunotherapy. 2024;16(4). doi:10.2217/imt-2023-0274
18. Boehringer Ingelheim. A study to test how well different doses of BI 771716 are tolerated by people with an advanced form of age-related macular degeneration (AMD) called geographic atrophy. February 18, 2025. https://clinicaltrials.gov/study/NCT06006585?term=bi%20771716&rank=1.
19. Schliefer, LS. FDA approval and successful launch of Eylea HD. Presentation at J.P.Morgan Healthcare Conference. January 8, 2024. https://regeneronpharmaceuticalsinc.gcs-web.com/static-files/7dfdabe2-05d1-4145-b5c5-342cec0ce9c4.
20. https://regeneronpharmaceuticalsinc.gcs-web.com/static-files/7dfdabe2-05d1-4145-b5c5-342cec0ce9c4. Accessed June 23, 2025.
21. Intravitreal AAVCAGsCD59 for advanced dry age-related macular degeneration (AMD) with geographic atrophy (GA). May 10, 2021. ClinicalTrials.gov ID NCT04358471 (GA). https://clinicaltrials.gov/show/NCT04358471. Accessed June 23, 2025.
22. Desai D, Dugel PU. Complement cascade inhibition in geographic atrophy: a review. Eye (Basingstoke). 2022;36(2). doi:10.1038/s41433-021-01765-x
23. A study to evaluate intravitreal JNJ-81201887 (AAVCAGsCD59) compared to sham procedure for the treatment of geographic atrophy (GA) secondary to age-related macular degeneration (AMD). May 10, 2021. https://clinicaltrials.gov/show/NCT05811351. Accessed June 23, 2025.
24. Heier JS, Cohen MN, Chao DL, et al. Phase 1 Study of JNJ-81201887 gene therapy in geographic atrophy secondary to age-related macular degeneration. Ophthalmol. 2024;131(12):1377-1388. doi:10.1016/j.ophtha.2024.06.013
25. Keefe D, Munye M, Hasan R, Rathi S, Bishop P, Clark S. CTx001, a gene therapy for the treatment of geographic atrophy in aged-related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64(8):3852.
26. FOCUS: A Phase I/II first in human study to evaluate the safety and efficacy of GT005 administered in subjects with dry AMD. https://www.clinicaltrials.gov/study/NCT03846193.
27. HORIZON: A phase II study to evaluate the safety and efficacy of two doses of GT005. https://clinicaltrials.gov/study/NCT04566445.
28. Gyroscope Therapeutics announces presentation of positive interim Phase I/II data for investigational gene therapy GT005 at Retina Society Annual Scientific Meeting. https://www.biospace.com/article/releases/gyroscope-therapeutics-announces-presentation-of-positive-interim-phase-i-ii-data-for-investigational-gene-therapy-gt005-at-retina-society-annual-scientific-meeting/.
29. Novartis. GT005 (PPY988): Development program in geographic atrophy. September 11, 2023 https://www.novartis.com/news/gt005-ppy988-development-program-geographic-atrophy. Accessed June 23, 2025.